For those unfamiliar with the term PMTA, it stands for Pre-Market Tobacco Application. It is a comprehensive document that must be submitted to the FDA that seeks to demonstrate that a vape product is "appropriate for the protection of the public health."
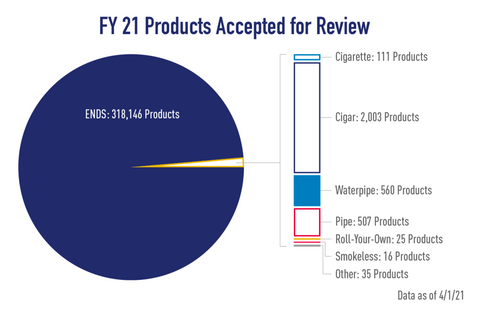
The PMTA process is far from easy, and it is often both time-consuming and expensive for manufacturers. Nevertheless, it is a necessary hurdle for any serious vaping company to clear if they want to keep their products on the market.
One of the most challenging aspects of the PMTA submission process is the scientific evidence required to support the application. Vape companies must provide a wealth of information on their products, including the ingredients, the manufacturing process, and the potential impact on public health.
It's no secret that the vaping industry has faced numerous challenges in recent years, including a rising number of bans and restrictions. However, despite the ongoing controversy surrounding vaping products, there is still a significant demand for these products, especially from former smokers looking for a healthier alternative to traditional cigarettes.
The PMTA process has undoubtedly put the industry to the test, separating legitimate vaping products from those that don't meet the necessary standards. As a result, there is hope that this will help to increase the credibility of the vaping industry and its products.
At its core, the PMTA requirement is designed to ensure that vaping products are safe for public consumption. There is no doubt that this level of scrutiny is necessary, especially when considering the health risks posed by traditional cigarettes.
The PMTA process has indeed been a controversial topic for many vape companies. However, it is essential to keep in mind that the overall goal is to protect the public's health. A rigorous and comprehensive application process is necessary to ensure that all vaping products meet the necessary standards.
While the PMTA deadline may result in some inconvenience for vape consumers, it is important to keep the broader perspective in mind. Vaping products are still a viable option for smokers looking to quit smoking, and the PMTA requirement will only help to provide greater assurances about the safety and quality of these products.
In conclusion, the recent PMTA submissions by vaping companies will undoubtedly have a significant impact on the industry. The application process is rigorous and extensive, but it is essential to ensure that vaping products are safe for public consumption. Despite the challenges, the deadline has led to a wave of submissions and improvements, increasing the credibility and legitimacy of the vaping industry as a whole. Vape PMTA could be the difference between a safe and healthy product or something that poses health risks to users.